
CHEBI:17283
| Name | 1-phosphatidyl-1D-myo-inositol 3-phosphate | 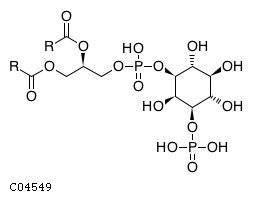 |
| Synonyms | 01,2-diacyl-sn-glycero-3-phospho-(1'-myo-inositol-3'-phosphate); 1-phosphatidyl-1d-myo-inositol 3-phosphate; 2,3-bis(alkanoxy)propyl (1s,2r,3s,4s,5r,6r)-2,3,4,6-tetrahydroxy-5-(phosphonatooxy)cyclohexyl phosphate; Phosphatidylinositol 3-phosphate; Pi3p; Pips; Ptdins(3)p; Ptdins-3-p; Ptdins3p; | |
| Definition | A phosphatidylinositol 3-phosphate in which the inositol moiety is the D-myo-isomer. | |
| Molecular Weight (Exact mass) | NA | |
| Molecular Formula | C11H18O16P2R2 | |
| SMILES | [H][C@@](COC([*])=O)(COP(O)(=O)O[C@H]1[C@H](O)[C@@H](O)[C@H](O)[C@@H](OP(O)(O)=O)[C@H]1O)OC([*])=O | |
| InChI | InChI=1S/C3H9O6P/c4-1-3(2-5)9-10(6,7)8/h3-5H,1-2H2,(H2,6,7,8) | |
| InChI Key | DHCLVCXQIBBOPH-UHFFFAOYSA-N | |
| Crosslinking annotations | KEGG:C04549 | ChEBI:17283 | PubChem:7155 |
| Pathway ID | Pathway Name | Pathway Description (KEGG) |
| map00562 | Inositol phosphate metabolism | NA |
| map01100 | Metabolic pathways | NA |
| map04070 | Phosphatidylinositol signaling system | NA |
| map04136 | Autophagy - other | Autophagy is a degradative pathway for the removal of cytoplasmic materials in eukaryotic cells, and is characterized by the formation of a double-membrane structure called the autophagosome, either in a housekeeping capacity or during stress and senescence. The process of autophagy could be divided into several stages: induction, vesicle nucleation, elongation and closure, and fusion and digestion. Most essential autophagic machineries are conserved throughout eukaryotes (see map04140 for animals and map04138 for fungi). This map is for other eukaryotes including plants and protists, where autophagy related genes (ATGs) play similar roles in the life cycle. However, autophagy has been relatively less studied in lower eukaryotes. |
| map04138 | Autophagy - yeast | Autophagy is a non-selective and bulk intracellular degradation system of eukaryotic cells and is highly conserved from yeast to human. In this process, the double-membrane vesicle, known as autophagosome, is formed and sequesters organelles or portions of cytosol. The autophagosome is subsequently fused with the vacuole for breakdown by resident hydrolases, and the resulting metabolites are delivered for reuse. In yeast, nutrient withdrawal is the primary stimulus that induces autophagy. Autophagy plays a central role in normal development and cell homeostasis of yeast. |
| map04140 | Autophagy - animal | Autophagy (or macroautophagy) is a cellular catabolic pathway involving in protein degradation, organelle turnover, and non-selective breakdown of cytoplasmic components, which is evolutionarily conserved among eukaryotes and exquisitely regulated. This progress initiates with production of the autophagosome, a double-membrane intracellular structure of reticular origin that engulfs cytoplasmic contents and ultimately fuses with lysosomes for cargo degradation. Autophagy is regulated in response to extra- or intracellular stress and signals such as starvation, growth factor deprivation and ER stress. Constitutive level of autophagy plays an important role in cellular homeostasis and maintains quality control of essential cellular components. |
| map04144 | Endocytosis | Endocytosis is a mechanism for cells to remove ligands, nutrients, and plasma membrane (PM) proteins, and lipids from the cell surface, bringing them into the cell interior. Transmembrane proteins entering through clathrin-dependent endocytosis (CDE) have sequences in their cytoplasmic domains that bind to the APs (adaptor-related protein complexes) and enable their rapid removal from the PM. In addition to APs and clathrin, there are numerous accessory proteins including dynamin. Depending on the various proteins that enter the endosome membrane, these cargoes are sorted to distinct destinations. Some cargoes, such as nutrient receptors, are recycled back to the PM. Ubiquitylated membrane proteins, such as activated growth-factor receptors, are sorted into intraluminal vesicles and eventually end up in the lysosome lumen via multivesicular endosomes (MVEs). There are distinct mechanisms of clathrin-independent endocytosis (CIE) depending upon the cargo and the cell type. |
| map04145 | Phagosome | Phagocytosis is the process of taking in relatively large particles by a cell, and is a central mechanism in the tissue remodeling, inflammation, and defense against infectious agents. A phagosome is formed when the specific receptors on the phagocyte surface recognize ligands on the particle surface. After formation, nascent phagosomes progressively acquire digestive characteristics. This maturation of phagosomes involves regulated interaction with the other membrane organelles, including recycling endosomes, late endosomes and lysosomes. The fusion of phagosomes and lysosomes releases toxic products that kill most bacteria and degrade them into fragments. However, some bacteria have strategies to escape the bactericidal mechanisms associated with phagocytosis and survive within host phagocytes. |
| map05152 | Tuberculosis | Tuberculosis, or TB, is an infectious disease caused by Mycobacterium tuberculosis. One third of the world's population is thought to be infected with TB. About 90% of those infected result in latent infections, and about 10% of latent infections develop active diseases when their immune system is impaired due to the age, other diseases such as AIDS or exposure to immunosuppressive drugs. TB is transmitted through the air and primarily attacks the lungs, then it can spread by the circulatory system to other parts of body. Once TB bacilli have entered the host by the respiratory route and infected macrophages in the lungs, they interfere with phagosomal maturation, antigen presentation, apoptosis and host immune system to establish persistent or latent infection. |

