
CHEBI:17553
| Name | O-phosphoethanolamine | 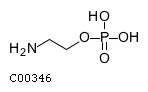 |
| Synonyms | 02-amino-ethanol dihydrogen phosphate; 2-amino-ethanol phosphate; 2-azaniumylethyl phosphate; Colamine phosphate; Colamine phosphoric acid; Colaminphosphoric acid; Eap; Ethanolamine acid phosphate; Ethanolamine o-phosphate; Ethanolamine phosphate; Ethanolamine?o-phosphate; Mono(2-aminoethyl) phosphate; Monoaminoethyl phosphate; O-phosphocolamine; O-phosphoethanolamine; O-phosphorylethanolamine; Ope; Pe; Pea; Petn; Phosphoethanolamine; Phosphonoethanolamine; Phosphoric acid 2-aminoethyl phenyl ester; | |
| Definition | The ethanolamine mono-ester of phosphoric acid, and a metabolite of phospholipid metabolism. This phosphomonoester shows strong structural similarity to the inhibitory neurotransmitter GABA, and is decreased in post-mortem Alzheimer's disease brain. | |
| Molecular Weight (Exact mass) | 141.063 (141.0191) | |
| Molecular Formula | C2H8NO4P | |
| SMILES | NCCOP(O)(O)=O | |
| InChI | InChI=1S/C2H8NO4P/c3-1-2-7-8(4,5)6/h1-3H2,(H2,4,5,6) | |
| InChI Key | SUHOOTKUPISOBE-UHFFFAOYSA-N | |
| Crosslinking annotations | KEGG:C00346 | 3DMET:B00092 | CAS:1071-23-4 | ChEBI:17553 | ChEMBL:CHEMBL146972 | NIKKAJI:J12.176B | PDB-CCD:OPE | PubChem:3639 |
| Pathway ID | Pathway Name | Pathway Description (KEGG) |
| map00564 | Glycerophospholipid metabolism | NA |
| map00600 | Sphingolipid metabolism | NA |
| map01100 | Metabolic pathways | NA |
| map01503 | Cationic antimicrobial peptide (CAMP) resistance | Cationic antimicrobial peptides (CAMPs) play an important role in host defense against microbial infection and are key components of the innate immune response. These are found among all classes of life ranging from prokaryotes to humans. In addition to the natural peptides, thousands of synthetic variants have been produced. CAMPs weaken the integrity of the bacterial inner and outer membranes and subsequently kill bacterial cells. On the other hand, bacteria have developed a number of mechanisms against CAMPs. These resistance mechanisms include decreased affinity to CAMPs by substitution of anionic cell surface constituents with cationic molecules; biosynthesis and crosslinking of cell envelope components; external trapping mechanisms that bind or neutralize the CAMPs by direct secretion of proteins, or the release of CAMPs binding molecules from the host cell surface; membrane efflux pumps; and production of peptidases. |
| map04071 | Sphingolipid signaling pathway | Sphingomyelin (SM) and its metabolic products are now known to have second messenger functions in a variety of cellular signaling pathways. Particularly, the sphingolipid metabolites, ceramide (Cer) and sphingosine-1-phosphate (S1P), have emerged as a new class of potent bioactive molecules. Ceramide can be generated de novo or by hydrolysis of membrane sphingomyelin by sphingomyelinase (SMase). Ceramide is subsequently metabolized by ceramidase to generate sphingosine (Sph) which in turn produces S1P through phosphorylation by sphingosine kinases 1 and 2 (SphK1, 2). Both ceramide and S1P regulate cellular responses to stress, with generally opposing effects. S1P functions as a growth and survival factor, acting as a ligand for a family of G protein-coupled receptors, whereas ceramide activates intrinsic and extrinsic apoptotic pathways through receptor-independent mechanisms. |

