
CHEBI:17665
| Name | alpha-D-glucose 6-phosphate | 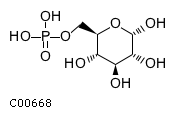 |
| Synonyms | 06-o-phosphono-alpha-d-glucopyranose; Alpha-d-glucose 6-phosphate; Alpha-d-glucose-6-phosphate; Alpha-d-glucopyranose 6-phosphate; | |
| Definition | A D-glucopyranose 6-phosphate where alpha-D-glucose is the sugar component. | |
| Molecular Weight (Exact mass) | 260.1358 (260.0297) | |
| Molecular Formula | C6H13O9P | |
| SMILES | O[C@H]1O[C@H](COP(O)(O)=O)[C@@H](O)[C@H](O)[C@H]1O | |
| InChI | InChI=1S/C6H13O9P/c7-3-2(1-14-16(11,12)13)15-6(10)5(9)4(3)8/h2-10H,1H2,(H2,11,12,13)/t2-,3-,4+,5-,6+/m1/s1 | |
| InChI Key | NBSCHQHZLSJFNQ-DVKNGEFBSA-N | |
| Crosslinking annotations | KEGG:C00668 | 3DMET:B01304 | ChEBI:17665 | NIKKAJI:J40.066A | PDB-CCD:G6P | PubChem:3937 |
| Pathway ID | Pathway Name | Pathway Description (KEGG) |
| map00010 | Glycolysis / Gluconeogenesis | Glycolysis is the process of converting glucose into pyruvate and generating small amounts of ATP (energy) and NADH (reducing power). It is a central pathway that produces important precursor metabolites: six-carbon compounds of glucose-6P and fructose-6P and three-carbon compounds of glycerone-P, glyceraldehyde-3P, glycerate-3P, phosphoenolpyruvate, and pyruvate [MD:M00001]. Acetyl-CoA, another important precursor metabolite, is produced by oxidative decarboxylation of pyruvate [MD:M00307]. When the enzyme genes of this pathway are examined in completely sequenced genomes, the reaction steps of three-carbon compounds from glycerone-P to pyruvate form a conserved core module [MD:M00002], which is found in almost all organisms and which sometimes contains operon structures in bacterial genomes. Gluconeogenesis is a synthesis pathway of glucose from noncarbohydrate precursors. It is essentially a reversal of glycolysis with minor variations of alternative paths [MD:M00003]. |
| map00030 | Pentose phosphate pathway | The pentose phosphate pathway is a process of glucose turnover that produces NADPH as reducing equivalents and pentoses as essential parts of nucleotides. There are two different phases in the pathway. One is irreversible oxidative phase in which glucose-6P is converted to ribulose-5P by oxidative decarboxylation, and NADPH is generated [MD:M00006]. The other is reversible non-oxidative phase in which phosphorylated sugars are interconverted to generate xylulose-5P, ribulose-5P, and ribose-5P [MD:M00007]. Phosphoribosyl pyrophosphate (PRPP) formed from ribose-5P [MD:M00005] is an activated compound used in the biosynthesis of histidine and purine/pyrimidine nucleotides. This pathway map also shows the Entner-Doudoroff pathway where 6-P-gluconate is dehydrated and then cleaved into pyruvate and glyceraldehyde-3P [MD:M00008]. |
| map00052 | Galactose metabolism | NA |
| map00520 | Amino sugar and nucleotide sugar metabolism | NA |
| map01060 | Biosynthesis of plant secondary metabolites | NA |
| map01061 | Biosynthesis of phenylpropanoids | NA |
| map01063 | Biosynthesis of alkaloids derived from shikimate pathway | NA |
| map01064 | Biosynthesis of alkaloids derived from ornithine, lysine and nicotinic acid | NA |
| map01065 | Biosynthesis of alkaloids derived from histidine and purine | NA |
| map01066 | Biosynthesis of alkaloids derived from terpenoid and polyketide | NA |
| map01070 | Biosynthesis of plant hormones | NA |
| map01100 | Metabolic pathways | NA |
| map01110 | Biosynthesis of secondary metabolites | NA |
| map01120 | Microbial metabolism in diverse environments | NA |
| map01130 | Biosynthesis of antibiotics | NA |
| map01200 | Carbon metabolism | Carbon metabolism is the most basic aspect of life. This map presents an overall view of central carbon metabolism, where the number of carbons is shown for each compound denoted by a circle, excluding a cofactor (CoA, CoM, THF, or THMPT) that is replaced by an asterisk. The map contains carbon utilization pathways of glycolysis (map00010), pentose phosphate pathway (map00030), and citrate cycle (map00020), and six known carbon fixation pathways (map00710 and map00720) as well as some pathways of methane metabolism (map00680). The six carbon fixation pathways are: (1) reductive pentose phosphate cycle (Calvin cycle) in plants and cyanobacteria that perform oxygenic photosynthesis, (2) reductive citrate cycle in photosynthetic green sulfur bacteria and some chemolithoautotrophs, (3) 3-hydroxypropionate bi-cycle in photosynthetic green nonsulfur bacteria, two variants of 4-hydroxybutyrate pathways in Crenarchaeota called (4) hydroxypropionate-hydroxybutyrate cycle and (5) dicarboxylate-hydroxybutyrate cycle, and (6) reductive acetyl-CoA pathway in methanogenic bacteria. |
| map04152 | AMPK signaling pathway | AMP-activated protein kinase (AMPK) is a serine threonine kinase that is highly conserved through evolution. AMPK system acts as a sensor of cellular energy status. It is activated by increases in the cellular AMP:ATP ratio caused by metabolic stresses that either interfere with ATP production (eg, deprivation for glucose or oxygen) or that accelerate ATP consumption (eg, muscle contraction). Several upstream kinases, including liver kinase B1 (LKB1), calcium/calmodulin kinase kinase-beta (CaMKK beta), and TGF-beta-activated kinase-1 (TAK-1), can activate AMPK by phosphorylating a threonine residue on its catalytic alpha-subunit. Once activated, AMPK leads to a concomitant inhibition of energy-consuming biosynthetic pathways, such as protein, fatty acid and glycogen synthesis, and activation of ATP-producing catabolic pathways, such as fatty acid oxidation and glycolysis. |
| map04922 | Glucagon signaling pathway | Glucagon is conventionally regarded as a counterregulatory hormone for insulin and plays a critical anti-hypoglycemic role by maintaining glucose homeostasis in both animals and humans. To increase blood glucose, glucagon promotes hepatic glucose output by increasing glycogenolysis and gluconeogenesis and by decreasing glycogenesis and glycolysis in a concerted fashion via multiple mechanisms. Glucagon also stimulates hepatic mitochondrial beta-oxidation to supply energy for glucose production. Glucagon performs its main effect via activation of adenylate cyclase. The adenylate-cyclase-derived cAMP activates protein kinase A (PKA), which then phosphorylates downstream targets, such as cAMP response element binding protein (CREB) and the bifunctional enzyme 6-phosphofructo-2-kinase/ fructose-2,6-bisphosphatase (one of the isoforms being PFK/FBPase 1, encoded by PFKFB1). |

